Sodium is a and will electrons when it forms an ion A metal lose 2 B nonmetal. Sodium is a _____ and will _____ electrons when it forms an ion. A) metal lose 2 B) nonmetal lose 1 C) metal gain 2 D) nonmetal gain 1 E) metal lose 1. Chem 1406 Quiz 1 Name: Aluminum phosphite B) Aluminum phosphate C) Aluminum phosphide D)Favorite Answer a. calcium will generally want to give two elections because it has 2 valence elections and a charge of +2 b. fluorine will generally want to gain one election because it has 7...Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H+ or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li+ Beryllium 4 2 Lose 2 Be2+ Boron 5 3 Aluminum 13 3 Lose 3 Al3+ Silicon 14 4 Gain or Lose 4 Si4+ or Si4-Phosphorus 15 5 Gain 3 P3-Sulfur 16 6 Gain 2 S2-It would tend to gain one electron and form a -1 ion. Aluminum is in the fifth column and therefore has 5 electrons in its outermost shell. It would tend to lose three electrons and form a +3 ion. How many electrons will Aluminum gain or lose when it forms an ion quizlet?How many electrons will aluminum gain or lose when it forms an ion? gain 5 gain 1 lose 1 lose 3 lose 2. lose 3. According to naming rules, the types of compound that use prefixes in their names are ionic compounds. compounds that contain polyatomic ions. covalent compounds.
How many electrons will each element gain or loose in
Answer to: How many electrons will aluminum gain or lose when it forms an ion? a. lose 1 b. lose 2 c. lose 3 d. gain 1 By signing up, you'll...How many electrons will lithium gain or lose when it forms an ion? asked Sep 16, 2016 in Chemistry by Judys. A) lose 1 B) gain 5 C) lose 2 D) lose 3 E) gain 1. general-chemistry; 0 Answers. 0 votes. answered Sep 16, 2016 by Buggy_boy . Best answer. A 0 votes. answered Sep 16It would tend to gain one electron and form a -1 ion. Aluminum is in the fifth column and therefore has 5 electrons in its outermost shell. It would tend to lose three electrons and form a +3 ion.2) How many electrons will aluminum gain or lose when it forms an ion? A) lose 1 B) gain 5 C) lose 2 D) lose 3 E) gain 1

PDF Table 1. Valence Electrons and Ion Formation for the First
science. chemistry. chemistry questions and answers. How Many Electrons Will Aluminum Gain Or Lose When It Forms An Ion?1. When a potassium atom reacts with a bromine atom, the bromine atom will a) lose 1 electron and form a positive ion b) lose 2 electrons and form a positive ion c) gain 1 electron and form a negative ion d) gain 2 electrons and form a negative ion I know . asked by Lisa on October 21, 2010; chemistryhow many electrons will aluminum gain or lose when it forms an ion? lose 3. the number of electrons in an ion with 16 protons and an ionic charge of -2 is. 18. elements in group 2a (2) of the periodic table form ions with a charge of how many electrons will chlorine gain or lose when it forms an ion? gain 1.How many electrons will aluminum gain or lose when it forms an ion? A. lose 1 B. gain 5 C. lose 2 D. lose 3 E. gain 1Name: Isabella Celis Homework #3 CHM1032 Chapter 6 Ionic and Molecular Compounds 1) In ionic compounds, metals lose their valence electrons to form positively charged cations. 2) How many electrons will aluminum gain or lose when it forms an ion? Lose 3 electrons. 3) What is the symbol for the ion with 19 protons and 18 electrons?
How many electrons will Aluminum gain or lose to form an ion?
It would tend to gain one electron and shape a -1 ion. Aluminum is within the 5th column and due to this fact has 5 electrons in its outermost shell. It would have a tendency to lose 3 electrons and form a +3 ion.
How many electrons will Aluminum gain or lose when it forms an ion quizlet?
Elements in Group 6A(16) lose 6 electrons when they shape ions. The ion of aluminum is Al³⁻.
How many electrons will be gain or lose when it forms an ion?
All Group 1 atoms can lose one electron to shape definitely charged ions. For instance, potassium atoms do this to shape ions with the same electron configuration because the noble fuel argon. Group 2 atoms lose two electrons to form positively charged ions.
[embedded content material]What happens when aluminum becomes an ion?
When an aluminum atom turns into an ion, it drops three electrons. Since there are only 10 electrons, their price is subtracted from the choice of protons, and the adaptation is a good 3. Therefore, an ion of aluminum has a good price of 3, proven as 3+.
How many electrons are gained or misplaced in aluminum?
Initially, the aluminum atom had a rate of +13 + (−13) = 0; in different words, its fee was neutral because of the equal numbers of protons and electrons. When it becomes an ion, it loses 3 electrons, leaving at the back of only 10.
Does aluminum turn out to be a cation or anion?
Aluminum, a member of the IIIA circle of relatives, loses three electrons to form a 3+ cation. The halogens (VIIA parts) all have seven valence electrons. All the halogens gain a unmarried electron to fill their valence power stage. And all of them form an anion with a unmarried unfavorable price.
You may well be : What is countable income for va pensionWhat can be required for aluminum to form the ion Al3+?
In a impartial atom, number of protons=choice of electrons. Hence on this case the collection of electrons in the impartial atom will be 13. Now, for the reason that atom carries +3 price therefore it will have 13-3=10 electrons. By the best way the atom mentioned this is Aluminium (Al) and thus the cation will be Al3+.
How many electrons will oxygen gain in forming an ion?
Oxygen has an electron arrangement of (2, 6) and needs to gain two electrons to fill the n=2 power degree and achieve an octet of electrons in the outermost shell. The oxide ion will have a charge of 2− as a result of gaining two electrons.
What is the method of the nitride ion?
The nitride ion's formula is N3-. Nitrogen atoms need 3 electrons to fill its outer valence shell, which is 2p. The nucleus of a nitrogen atom
How many electrons does potassium need to lose to change into an ion?
Potassium needs to lose 1 electron to develop into an ion. It will become undoubtedly charged cation.
Is a cation?
A cation is a undoubtedly charged ion with fewer electrons than protons while an anion is negatively charged with extra electrons than protons, on account of their reverse electrical fees; cations and anions draw in every other and readily shape ionic compounds.
Why does sulfur form a 2 ion?
Sulfur has 16 electrons. To be isoelectronic with argon, which has 18 electrons, sulfur will have to gain two electrons. Therefore sulfur will form a 2– ion, changing into S2–.
How does the dimensions of an aluminum atom trade when it turns into an ion?
How does the dimensions of an aluminum atom charge when it becomes an ion with a rate of 3+? Positive ions decreases in measurement by losing electrons. Since aluminum turns into an ion with a rate of three+, it is shedding 3 electrons which reasons aluminum to lower its radius/ size.
You may well be : Quick Answer: When does lent officially finish?Why does Aluminum lose 3 electrons?
Aluminum is a Group IIIA part which means that it has 3 electrons in it's outer maximum shell, different sensible known as every atom of aluminum has Three valence electrons. Therefore aluminum atoms will lose 3 electrons so it takes on a +Three fee when it turns into ion.
Is oxygen a cation or anion?
Oxygen always has a price of adverse 2, so it is an anion. Oxygen is an uncharged molecule (O2). The standard oxygen ion is oxide, O- -, which is negatively charged, subsequently an anion.
Chapter 5..docx - 5 Multiple-Choice Questions 1 The Number Of Valence Electrons Found In An Atom Of A Group A Element Is Equal To A Its Atomic Number B | Course Hero

Chemistry Chap 3 Flashcards | Quizlet

Practice Exam 5 On Compounds And Their Bonds | CHEM 1152K - Docsity

Solved: Polzeomic Ions Oiate (РОУ)_ Ph Sphate (soy)- Sulfa... | Chegg.com

CH104: Chapter 3 – Ions And Ionic Compounds – Chemistry

Valence Electrons Are The Highest Energy S And P Electrons. Valence Electrons Are The Electrons Available For Bonding. An Electr
Valence Electron - Wikipedia

Chapter 4 – Ionic Compounds 1) In An Electron

Solved: 1. The Name Of AI2C04)) İS A) Aluminum(III) Sulfat... | Chegg.com

Untitled
Valence Electron - Wikipedia
Solved: 13. What Is The Name Of K2S? B) Potassium Disulfid... | Chegg.com

CH104: Chapter 3 – Ions And Ionic Compounds – Chemistry

Notebook Printing...
Untitled
Student Exploration Sheet: Growing Plants
CHM1032 Assignment 6.docx - CHM1032 Assignment 6 Multiple-Choice Questions 1 The Octet Rule Indicates That A All Of The Noble Gases Have Eight Total | Course Hero

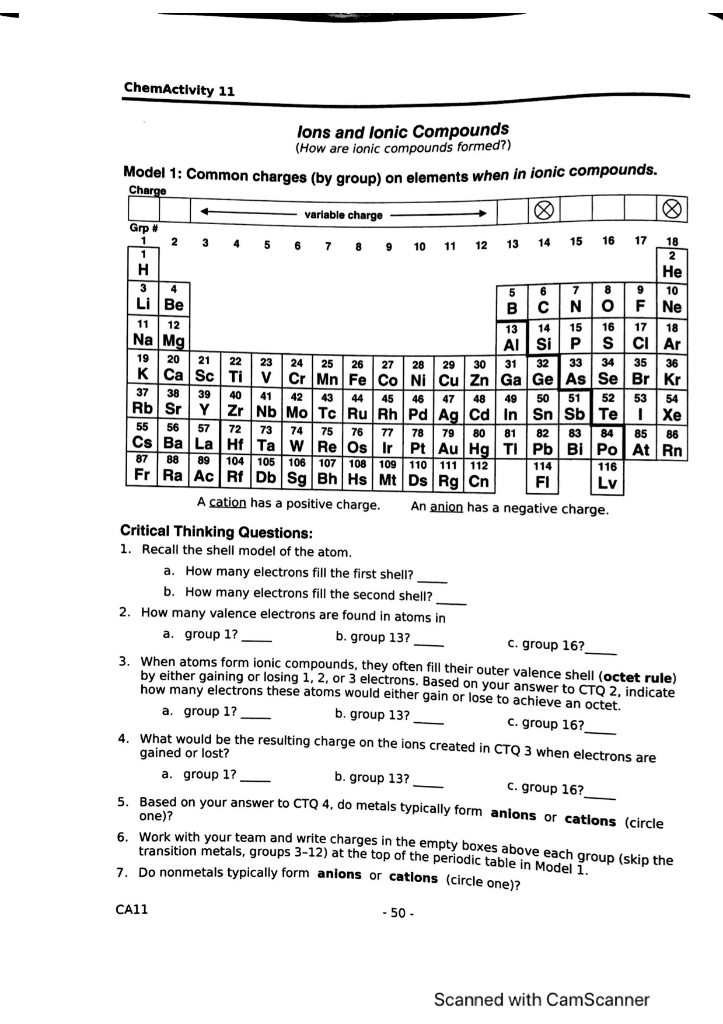
Solved: ChemActivity 11 2 M& 41 43 45 46 49 53 Lons And Lo... | Chegg.com
Solved: Rorce Completion This Test Can Be Saved And Resume... | Chegg.com

Lecture 4 Atoms And Atomic Structure Protons, Neutrons And Electrons The Atom Is The Smallest Building Block Of An Element. The Atom Is Composed Of A Core Called The Nucleus Where Most Of The Atoms Mass Is Concentrated. The Nucleus Is Composed Of Two

0 comments:
Post a Comment